
EMA Marketing Authorization of New Drugs in October 2024
Shots:
-
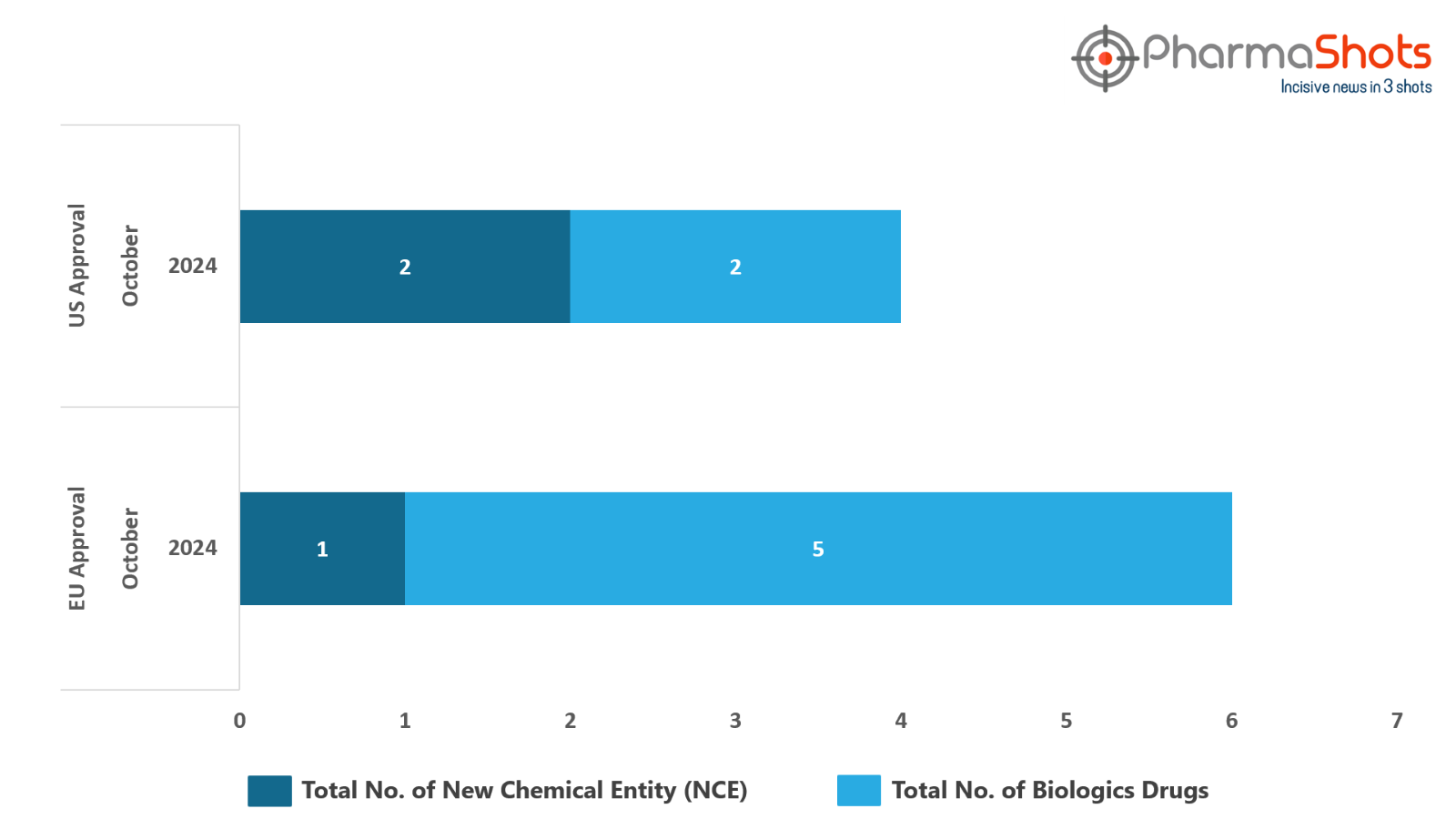
The EMA’s CHMP has granted positive opinions to 5 Biologics and 1 New Chemical Entity in October 2024, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were Novo Nordisk’s Alhemo to treat Haemophilia A or B with inhibitors and AstraZeneca & Ionis’ Wainzua for Hereditary Transthyretin-Mediated Amyloidosis
-
PharmaShots has compiled a list of 4 drugs that have been granted positive opinion by the EMA’s CHMP
Product Name: Wainzua
Active ingredient: Eplontersen
Company: AstraZeneca & Ionis
Date: Oct 17, 2024
Disease: Hereditary Transthyretin-Mediated Amyloidosis
Shots:
-
The opinion of Wainzua for ATTRv-PN (stage 1/2 polyneuropathy) was supported by its P-III (NEURO-TTRansform) study vs external PBO for over wk.66, with a follow-up until wk.85 & an end-of-trial evaluation. Eligible patients could then enter an ongoing OLE study
-
Study depicted sustained benefits in co-1EPs of serum transthyretin (TTR) levels & neuropathy impairment (mNIS+7) as well as 2EP of QoL (Norfolk QoL-DN) over 66wks., with a favorable & tolerable safety
-
Eplontersen is under P-III (CARDIO-TTRansform) trial for transthyretin-mediated amyloid cardiomyopathy with 1,400 subjects. Both the companies are commercializing Wainzua for ATTRv-PN in the US & pursuing approval in the EU & ROW (AstraZeneca holds exclusive rights)
Product Name: Siiltibcy
Active ingredient: Mycobacterium tuberculosis derived antigens (rdESAT-6 and rCFP-10)
Company: Serum Institute of India
Date: Oct 17, 2024
Disease: Mycobacterium tuberculosis
Shots:
-
The CHMP has recommended Siiltibcy (0.5μg/mL rdESAT-6 & rCFP-10) to diagnose Mycobacterium tuberculosis infection in individuals (age: ≥28 days), valid in the EU plus Norway, Iceland & Liechtenstein. Separate MAA will be submitted to the UK MHRA
-
Siiltibcy’s sensitivity & specificity was assessed in comparison with QuantiFERON TB Gold In-Tube test (QFT, in-vitro test) & Tuberculin purified derivative (PPD RT23, an intradermal test)
-
Serum Life Science Europe will be the MAA holder, Bilthoven Biologicals will handle import, release & commercialize Siiltibcy in the EU and Serum Institute of India will oversee manufacturing & regulatory compliance globally under an alliance b/w them
Product Name: Korjuny
Active ingredient: Catumaxomab
Company: Lindis Biotech
Date: Oct 17, 2024
Disease: Malignant Ascites
Shots:
-
The CHMP has granted a positive opinion to Korjuny (trifunctional anti-CD3 x anti-EpCAM Ab) for treating malignant ascites in adults with EpCAM+ carcinomas, not eligible for systemic anti-cancer treatment. EC’s decision is anticipated by YE’24, & will be valid in the EU plus Norway, Iceland & Liechtenstein
-
The opinion was based on P-II/III (IP-REM-AC-01) study showing four-fold increase in the 1EP of puncture-free survival vs therapy with only puncture treatment
-
Moreover, recruitment for the P-I (CATUNIBLA) dose escalation & expansion study for high and intermediate-risk non-muscle invasive bladder cancer (HR-NMIBC) has been completed. Interim readouts were highlighted at the ESMO 2024
Product Name: Alhemo
Active ingredient: Concizumab
Company: Novo Nordisk
Date: Oct 17, 2024
Disease: Haemophilia A or B
Shots:
-
The CHMP has recommended Alhemo (QD, SC) as a prophylactic treatment of hemophilia A/B with inhibitors in patients (≥12yrs.), with the EC’s decision anticipated within ~2mos.
-
The opinion was based on the P-III (explorer7) study assessing the efficacy & safety of Alhemo to treat haemophilia A or B with inhibitors. Alhemo will be available in a convenient, pre-mixed, and prefilled pen upon approval
-
Alhemo (concizumab) is a mAb that blocks anti-tissue factor pathway inhibitor (TFPI) for thrombin production, preventing blood clotting
Note: The following drugs have also been recommended for approval, however, no PR was available:
-
Fluad
-
Flucelvax
Related Post: Insights+: EMA Marketing Authorization of New Drugs in September 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














